Liquid Bromine Is Added to a Solution of Potassium Iodide
Magnesium chloride sodium fluoride magnesium fluoride sodium. Br 2 l 2I aq 2Br aq I 2 aq E θ cell 053 V.

How To Balance Ki Br2 Kbr I2 Potassium Iodide Bromine Gas Youtube
5 Liquid bromine is added to an aqueous solution of potassium iodide.

. Solid copper II sulfide is strongly heated in oxygen gas. Br2 2I- 2Br- I2. Check the balance Lithium iodide react with bromine to produce lithium bromide and iodine.
Solid calcium is added to warm water. Liquid bromine is added to a solution of potassium iodide. Br2 2NaI.
Liquid bromine is carefully added to a solution of potassium iodide Sr Cu2 Sr2 Cu A bar of strontium metal is immersed in a 10 M copper II nitrate solution. Periodic table of elements. Bromine is added to a solution of sodium iodide sodium iodide- sodium bromide iodine bromine Write the balanced chemical equation for this reaction.
The solution is usually added to water fruit juice or milk before drinking. Liquid bromine is added to a potassium iodide solution. This is an example of a displacement reaction where the more reactive element will replace a less reactive one.
It is mainly used in the form of a saturated solution 100 g of potassium iodide to 100 ml of water. Add your answer and earn points. B Chlorine is added to potassium iodide.
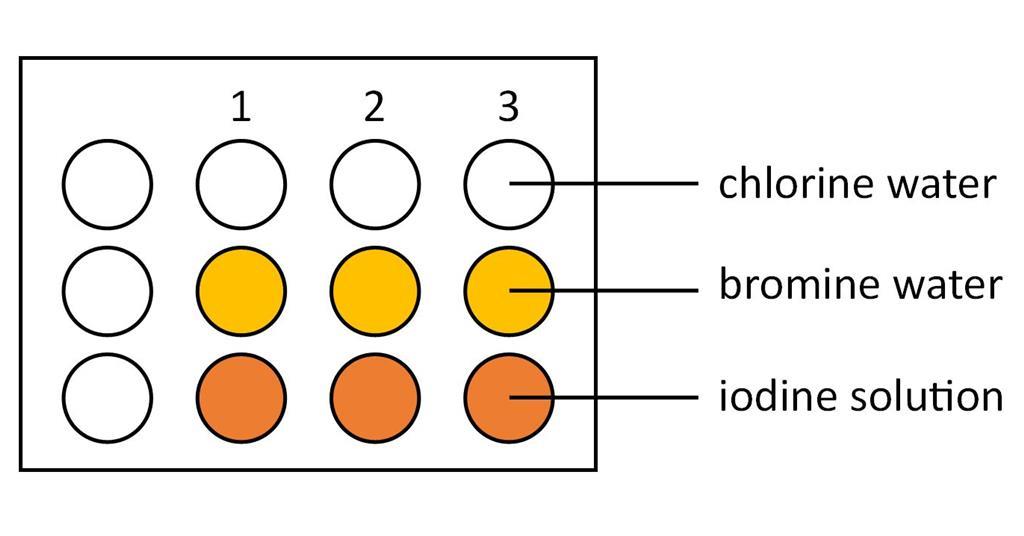
Bromine Water is is a pale yelloworange colour. Chlorine gas is bubbled into a solution of potassium iodide. Br2 I - Æ Br- I2 11.
Magnesium turnings are added to a solution of ironIII chloride. Aqueous solutions of barium chloride and ammonium sulfate are mixed. This equates to approximately 50 mgdrop.
Discuss the effect of concentration of a solution on standard electrode potential. 5 7Classify the following reaction. Chlorine gas is bubbled into a solution of sodium bromide.
Liquid bromine is added to a solution of potassium iodide. Potassium iodide KI is prepared by reacting iodine with a hot solution of potassium hydroxide. In this reaction the solution will have a slight change in color to a different shade of Brown since Bromine and Iodine solution are both in a different shade of brown.
In this case we have two halogens Bromine and Fluorine. Asked By Wiki User. B Draw a labelled diagram to show two linked half-cells that could be used to measure the standard cell potential for this reaction.
O Which substance is the oxidizing agent. Ethyene acetylene is burned in air. Write two half-equations for this reaction.
Pb 2H SO42- PbSO4 H2. Liquid bromine is carefully added to a solution of potassium iodide. Solid zinc strips are added to a solution of copperII sulfate.
Lead foil is immersed in silver nitrate solution. For example when bromine gas is bubbled through a solution of potassium iodide in water the more reactive bromine will displace take the place of the less reactive iodide forming iodine and potassium bromide. Draw a labelled diagram to show two linked half-cells that could be used to measure the standard cell potential for this reaction.
As an ionic equation. Lead foil is immersed in silver nitrate solution. Br 2 2KI I 2 2KBr.
Liquid bromine is added to an aqueous solution of potassium iodide. Solid zinc strips are added to a solution of copperII sulfate. Liquid bromine is added to a solution of potassium iodide.
I interpreted it with this molecular formula ceBr_2l 2NaI_s rightarrow 2NaBr_s I_2g. Answer 1 of 3. Bromine potassium iodide iodine potassium bromide.
Can liquid bromine react with potassium iodide. Thermodynamic properties of substances. The reaction would go brown because chlorine is more reactive than iodine.
Zn Cu2 Æ Zn2 Cu 14. Cl I - o Cl - I. From my understanding since none of the products nor any of the reactants is aqueous there shouldnt be a net ionic equation.
Hot hydrogen gas is passed over heated copper II oxide solid. Ethanol is burned in oxygen gas. The solubility of the substances.
Barium reacts with nitrogen. Why bromine solution added gradually. Cl 2 Br 2 o 2Cl - 2Br - This reaction happens because chlorine is smaller than bromine so it is more reactive.
Liquid bromine is added to a container of sodium iodide crystals. Where do the smoke and gases go after the fire. Cl2 I-Æ Cl- I2 12.
Cl2 2Br- 2Cl- Br2. Sodium metal is added to water. Magnesium turnings are added to a solution of iron III chlor Describe the color change that corresponds to the read.
Hexane is combusted in air. Ca H2O Æ Ca2 OH- H2 10. Reactions-practice-allpdf - Read File Online - Report Abuse.
When it is added to potassium iodide solution it oxidises the iodide ion to iodine which is also coloured - forming a brown solution Hence one might notice an intensification of colour. Kc SO32 SO22 O2. Chlorine gas is bubbled into a solution of potassium iodide.
Pellets of lead are dropped into hot sulfuric acid. KI and KBr are both colorless its the Br2 and I2 that has color 397K views. What is the sum of the coefficients simplest whole number ratio.
Br2 l 2 KI aq 2 KBr aq I2. Liquid bromine is added to a solution of potassium iodide. Cyclohexane would be purple.
The following reaction takes place. As we already have an ionic compound KF between Potassium and Fluorine the Bromine will attempt to replace the Fluorine to form Potassium bromide KBr - but it will fail to. Pb Ag Æ Pb2 Ag 13.
Yes it can by displacing the Iodide. Liquid bromine is added to a solution of potassium iodide. Lead foil is immersed in silver nitrate solution.
Propene is burned in air. 2LiI Br 2 2LiBr I 2. Chlorine gas is bubbled into a solution of potassium iodide.
Mg Fe3 3Cl- MgCl2 Fe. What is the balanced chemical equation 1 See answer imankhan6288 is waiting for your help. The following reaction takes place.
Br_0 21 aq 2Br aq I_ aq 053V a Write two half-equations for this reaction. Pure liquid bromine is added to a zinc iodide solution.

Solved Liquid Bromine Is Added To An Aqueous Solution Of Potassium Iodide The Following Reaction Takes Place Brz L 21 Aq 2br Aq T Aq Ec4l 0 53v Write Two Halt Equations Tor This Reaction

Bromine And Potassium Iodide Youtube
Halogens In Aqueous Solution And Their Displacement Reactions Experiment Rsc Education
Comments
Post a Comment